Hamza Asumah, MD, MBA
The healthcare industry is a complex and dynamic landscape where innovation is not just celebrated but necessary. For healthcare entrepreneurs, the path to a successful product launch is fraught with unique challenges and regulatory hurdles that can be daunting. However, with a strategic approach encapsulated by thorough market research, meticulous regulatory planning, well-executed pilot studies, and robust marketing strategies, success is within reach. This comprehensive guide will delve into the multi-step process of introducing a healthcare product to the market, backed by relevant data and proven principles that can assist entrepreneurs in making informed decisions.
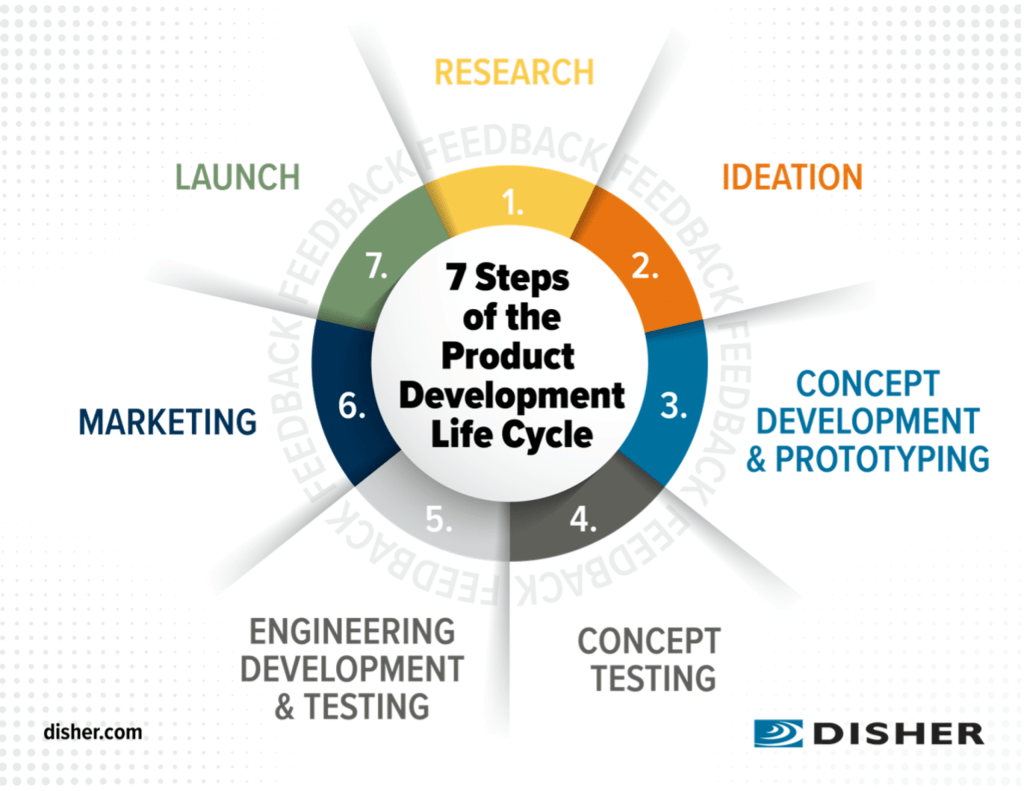
Photo By Disher
Step 1: Inception and Market Research
The journey of launching a healthcare product starts with an idea. However, an idea alone is insufficient without understanding the market landscape. Comprehensive market research is essential for several reasons:
Identifying Target Market: Entrepreneurs must identify who needs the product, the size of the target market, and how the product will meet specific healthcare needs.
Analyzing Competition: Understanding the strengths, weaknesses, and market strategies of competitors allows for differentiation and niche targeting.
Assessing Market Trends: Knowledge of current and future trends in healthcare can shape product development to align with industry direction.
Relevant Data: According to a report by the Business Market Research Collection, over 80% of new products fail due to a lack of understanding of market needs. Market research can reduce this risk significantly.
Photo By Concept Board
Step 2: Regulatory Roadmap and Approval
Healthcare products must adhere to strict regulatory requirements. The process can vary significantly depending on the region and type of product (medical device, pharmaceutical, health IT, etc.). Key activities include:
Identifying Relevant Regulations: Entrepreneurs must be familiar with FDA regulations in the US, EMA in Europe, or other regulatory bodies appropriate for their market.
Quality Management Systems: Implementation of systems like ISO 13485 for medical devices is crucial for meeting regulatory standards.
Clinical Trials and Approvals: Products may require pre-clinical and clinical trials to demonstrate safety and efficacy.
Relevant Data: FDA data shows that the average time from submission to approval for medical devices is 177 days under the 510(k) process and 1,027 days for the PMA process. Planning for these timeframes is critical.
Step 3: Pilot Studies and Proof of Concept
Before a full-scale launch, pilot studies or limited market tests are invaluable. They provide:
Evidence of Efficacy and Safety: Small-scale trials can help validate the product’s effectiveness and identify any potential side effects or risks.
Feedback for Improvement: Responses from initial users can offer insights into product refinement and user experience.
Data for Investors: Positive results from pilot studies can attract additional funding and support.
Relevant Data: A study in the Journal of Product Innovation Management found that products tested through pilot studies have a 65% higher chance of success in the market.
Photo By PolarSeal
Step 4: Marketing Strategies and Launch Planning
With regulatory approval and successful pilot studies, the focus shifts to marketing and launch. A multifaceted marketing strategy should include:
Branding and Positioning: Establishing a strong brand identity and clear product positioning to distinguish from competitors.
Education and Outreach: Educating healthcare providers, payers, and patients about the product’s benefits is vital for adoption.
Multi-Channel Marketing: Leveraging digital marketing, social media, conferences, and industry events for broader reach.
Relevant Data: The Content Marketing Institute reveals that healthcare marketers using multi-channel marketing have seen an 89% success rate in achieving their organization’s goals.
Step 5: Post-Launch Assessment and Growth
After the launch, continuous monitoring and improvement are imperative. This phase involves:
Sales and Market Feedback: Tracking sales data and customer feedback to gauge the product’s market performance.
Post-Market Surveillance: Ongoing safety and efficacy monitoring is often a regulatory requirement and provides real-world data.
Scaling Up: Based on initial success, strategies for scaling production, distribution, and marketing efforts should be developed.
Relevant Data: According to McKinsey & Company, companies that invest in customer experience post-launch see a 20-30% increase in customer satisfaction and economic gains of 20-50%.

Photo By Philips
Launching a healthcare product is an intricate process that requires a well-structured plan and commitment to quality, safety, and market needs. By adhering to these outlined steps, healthcare entrepreneurs can not only increase their chances of a successful product launch but also make significant contributions to the wellbeing of patients and the healthcare system at large. As the industry evolves, staying agile and informed will continue to be critical for ongoing success.
Please leave your comments below

Leave a comment